Click the “Start Activity” button to indicate you have reviewed the CME information for this activity.
Activity Speakers
Stephan A. Grupp, MD, PhD
Program Chair
Director of the Cancer Immunotherapy Program
Director of Translational Research for the Center for Childhood Cancer Research
Medical Director of the Stem Cell Laboratory
The Children’s Hospital Philadelphia (CHOP)
Philadelphia, PA
Helen E. Heslop, MD, DSc (Hon)
Professor, Departments of Medicine and Pediatrics
Section of Hematology-Oncology
Dan L. Duncan Chair, Baylor College of Medicine
Interim Director, Center for Cell and Gene Therapy
Associate Director Clinical Research, Dan L. Duncan Cancer Center
Baylor College of Medicine
Director, Adult Stem Cell Transplant Program
Houston Methodist
Houston, TX
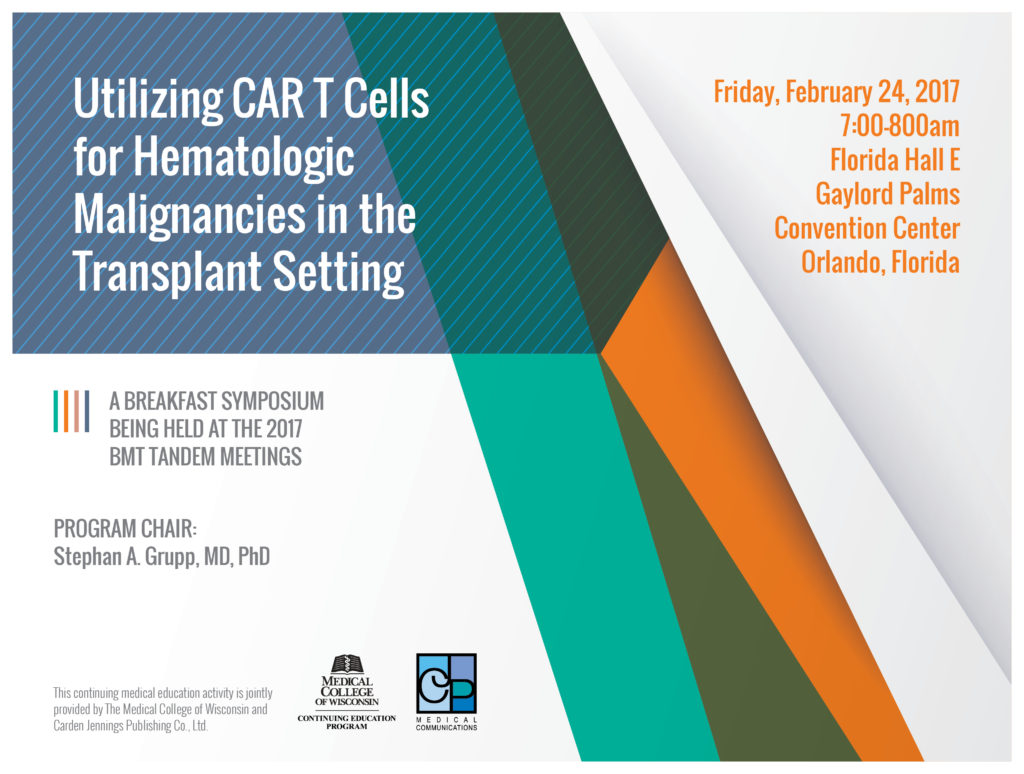
Release date: May 16, 2017
Expiration date: May 16, 2018
CME and Support Information
This continuing medical education activity is provided by The Medical College of Wisconsin. Collaborative Partner: Carden Jennings Publishing.
This activity is supported by educational grants from Novartis Pharmaceuticals and Kite Pharma.
Program Overview
The goal of this educational program is to improve the treatment of patients with hematologic malignancies through the dissemination of information about treating these diseases with CAR T cell therapy. This immunotherapy option appears to be on the brink of approval for certain blood related cancers, which may change the prognosis of patients with these disorders drastically. CAR T cell therapy has shown to bring patients with difficult to treat cancers to remission more quickly and effectively than most previous treatment options. CAR T cells may drastically change the treatment of hematologic malignancies, and this program serves as a medium to give health care professional a chance to recognize these changes.
Learning Objectives
Upon successful completion of this educational activity, participants should be better able to:
- Address safety concerns in the use of CAR T cell therapy
- Evaluate the promising CD19 CAR T cell options in clinical trials
- Discuss the issues that can arise after anti-CD19 CAR T cell therapy the potential impact on CAR T cell therapy
Target Audience
The intended audience for this activity is hematologists, oncologists, bone marrow transplant specialists, and other health care professionals who provide care for patients with hematologic malignancies.
Accreditation Statement
The Medical College of Wisconsin is accredited with commendation by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
AMA Credit Designation
The Medical College of Wisconsin designates this live activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity. This enduring material is approved for 1 year from the date of original release, May 15, 2017 to May 15, 2018.
Faculty and Planner Disclosures
In accordance with the Accreditation Council for Continuing Medical Education’s Standards for Com¬mercial Support, all CME providers are required to disclose to the activity audience the relevant financial relationships of the planners, teachers, and authors involved in the development of CME content. An individual has a relevant financial relationship if he or she has a financial relationship in any amount occurring in the last 12 months with a commercial interest whose products or services are discussed in the CME activity content over which the individual has control. Relationship information appears below:
Stephan A. Grupp, MD, PhD discloses that he has been a consultant, speaker, and has received research funding from Novartis.
Helen E. Heslop, MD, DSc (Hon) discloses that she has ownership interest in Viracyte and Marker Therapeutics. She has intellectual property rights and has been on the Advisory Board for Cell Medica. She also has received research funding from Celgene.
The educational content of this program has been peer reviewed by The Medical College of Wisconsin.
The Medical College of Wisconsin and Carden Jennings Publishing report the following relationship(s): No relevant financial relationships to disclose.
Signed disclosures are on file at The Medical College of Wisconsin.
Unlabeled and Investigational Usage
The audience is advised that this continuing education activity may contain references to unlabeled uses of FDA-approved products or to products not approved by the FDA for use in the United States. The faculty members have been made aware of their obligation to disclose such usage.
Disclaimer
The material presented at or in any Medical College of Wisconsin or Carden Jennings Publishing Company, Ltd. continuing education activity does not necessarily reflect the views and opinions of Medical College of Wisconsin or Carden Jennings Publishing. Neither Medical College of Wisconsin or Carden Jennings Publishing, nor the faculty endorse or recommend any techniques, commercial products, or manufacturers. The faculty/authors may discuss the use of materials and/or products that have not yet been approved by the U.S. Food and Drug Administration. All readers and continuing education participants should verify all information before treating patients or utilizing any product.
Click the “Start Activity” button to indicate you have reviewed the CME information for this activity.